7795ss01
7795ss01.docx
Bilingual Pesticide Labeling Tracking (NEW)
OMB:
Supporting
Statement for an Information Collection Request (ICR)
Under
the Paperwork Reduction Act (PRA)
EXECUTIVE SUMMARY
Identification of the Information Collection – Title and Numbers
Title: |
Bilingual Pesticide Labeling Tracking |
EPA ICR No.: |
7795.01 |
OMB Control No.: |
2070-NEW |
Docket ID No.: |
EPA-HQ-OPP-2025-0049 |
Abstract
This is a new information collection activity that covers the paperwork burden for tracking the adoption of bilingual labeling of pesticide products. This ICR was developed as part of a requirement by the Pesticide Registration Improvement Act (PRIA). PRIA was enacted in 2004 and established a new system for registering pesticides including fees and guaranteed decision times, along with funding for farmworker protection activities. PRIA was reauthorized in 2007, 2012, 2019, and most recently on December 29, 2022 (PRIA 5). PRIA 5 amended the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) to require Spanish language translation for parts of the end-use pesticide product labeling where translation is available in the EPA Spanish Translation Guide for Pesticide Labeling (or the Spanish Translation Guide). The Spanish Translation Guide contains translations of the following parts of pesticide product labeling—the “keep out of reach of children” statement, the restricted use pesticide statement for restricted use products, misuse statements, the signal word, first aid statements, the precautionary statements, personal protective equipment, engineering controls, environmental hazards, physical or chemical hazards, and the storage and disposal statements.
PRIA 5 requires that each registered pesticide product released for shipment include either the Spanish language translation for parts of the labeling contained in EPA’s Spanish Translation Guide for Pesticide Labeling on the pesticide product container, or a link to such translation via scannable technology or other electronic methods readily accessible on the product label. Antimicrobial pesticide products and non-agricultural/non-restricted use pesticide products may, in lieu of including a translation or a link to the translation, provide a link to the Spanish safety data sheets (SDS) via scannable technology or other electronic methods readily accessible on the product label.
PRIA 5 establishes a rolling schedule for the implementation of bilingual labeling, from December 2025 to 2030, with the translations for the most hazardous and toxic pesticide products required first. For Restricted Use Pesticides (RUPs) and agricultural pesticides classified as Acute Toxicity Category I, the deadline is three years after the enactment of PRIA 5, or December 29, 2025. For agricultural pesticides classified as Acute Toxicity Category II, the deadline is five years after enactment, or December 29, 2027. For non-agricultural pesticides classified as Acute Toxicity Category I the deadline is four years after enactment, or December 29, 2026, and those classified as Acute Toxicity Category II six years after enactment, or December 29, 2028. All other pesticides must have the relevant language translated by December 29, 2030. These changes to the pesticide product labeling are made using “non-notification” procedures, which means that the changes can be made without notifying, or submitting, the change to EPA for review.
PRIA 5 also requires EPA to develop, implement, and make publicly available a plan for tracking the adoption of bilingual labeling by December 29, 2024. The Summary Table below provides estimates of the average annual burden to both respondents and EPA. The actual annual burden changes for each subsequent year of the ICR’s three-year term and reflects the additional products that are scheduled to comply with PRIA 5 in each of the subsequent years according to the schedule.
Summary Table: Annual Total Annual Burden and Costs
Information Collection |
Number of Respondents |
Annual Number of Responses |
Responses per Respondent |
Annual Time Burden (Hours) |
Annual Cost Burden (Dollars) |
Bilingual Labeling Tracking Total Annual Respondent Burden |
1,517 |
4,631 |
4 |
27,784 |
$3,648,965 |
Total Annual Agency Burden |
|
|
|
42 |
$24,625 |
Source: EPA Estimate. Average 3-year Respondent Burden from Tables 3 and 4. Average 3-year Agency Burden estimate from Table 6.
SUPPORTING STATEMENT
1. Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
Pesticide registration activities are governed by the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), 7 U.S.C. 136 et seq., which was amended by the Pesticide Registration Improvement Act of 2022 (PRIA 5) on December 29, 2022. PRIA 5 amended FIFRA section 3(f)(5) to require parts of pesticide product labeling to be translated into Spanish. PRIA 5 provides deadlines for bilingual labeling to appear on pesticide products on a rolling schedule from December 29, 2025, to December 29, 2030, with translations for the most hazardous and toxic pesticide products required first. EPA is required by PRIA 5 to track the adoption of bilingual labeling (Ref. 1).
EPA published a proposed Pesticide Registration Notice (PRN) outlining its plan to track bilingual labeling compliance and received four comments. The response to those comments is included as Attachment B.
2. Indicate how, by whom, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the Agency has made of the information received from the current collection.
The information will be used by EPA to track the adoption of bilingual pesticide labeling, which is a requirement under PRIA 5. This is a new collection.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also describe any consideration of using information technology to reduce burden.
The information collection will be electronic. The information will be collected through the Agency’s MyPeST application. Pesticide companies are able to create free MyPeST accounts that provide access to the status of various actions at EPA for their pesticide products. The MyPeST application will be updated to allow companies to indicate whether their products have the required bilingual labeling translations by clicking on a checkbox. This update will occur by December 29, 2025. This process will minimize the burden of informing the Agency through a non-electronic process.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purposes described in Item 2 above.
There is no other source for information on whether bilingual labeling has been adopted by registrants, and bilingual labeling requirements begin in 2025. PRIA 5 has specified that these changes to the labeling may be made through non-notification, meaning that registrants are not required to submit the labeling to EPA.
5. If the collection of information impacts small businesses or other small entities, describe the methods used to minimize burden.
Many respondents are small businesses. The burden on these small businesses, as well as larger businesses, is minimized by making the information collection process electronic rather than requiring the submission of hardcopy forms with the bilingual labeling tracking information.
The Agency’s process to collect information via the MyPeST application requires the minimum amount of information needed to comply with the requirements in PRIA 5. The needs of small businesses were of primary concern in this approach to data collection. The respondents are asked to provide only readily available information.
6. Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
If the information is not collected, EPA will be unable to comply with the statutory requirements in PRIA 5 that adoption (end-use pesticide product labeling must be revised to include bilingual labeling for parts of the labeling where translation is available in the EPA Spanish Translation Guide for Pesticide Labeling) of bilingual labeling be monitored. Information will be collected annually because that corresponds with the rolling schedule outlined in PRIA 5.
7. Explain any special circumstances that require the collection to be conducted in a manner inconsistent with OMB guidelines.
a) requiring respondents to report information to the agency more often than quarterly;
b) requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
c) requiring respondents to submit more than an original and two copies of any document;
d) requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records, for more than three years;
e) in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
f) requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
g) that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
h) requiring respondents to submit proprietary trade secrets, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
There are no special circumstances. The collection of information is conducted in a manner consistent with the guidelines in 5 CFR 1320.5(d)(2).
8. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency’s notice, required by 5 CFR 1320.8(d), soliciting comments on the information collection prior to submission to OMB. Summarize public comments received in response to that notice and describe actions taken in response to the comments. Specifically address comments received on cost and hour burden.
Describe efforts to consult with persons outside EPA to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting format (if any), and on the data elements to be recorded, disclosed, or report.
Consultation with representatives of those from whom information is to be obtained or those who must compile records should occur at least once every 3 years - even if the collection of information activity is the same as in prior periods. There may be circumstances that may preclude consultation in a specific situation. These circumstances should be explained.
Pursuant to 5 CFR 1320.8(d), on July 21, 2025, EPA published a notice in the Federal Register, announcing the beginning of this information collection activity, soliciting public comment on specific aspects of the ICR, and providing a 60-day public comment period. EPA received two public comments in response to its notice that were generally supportive of the information collection, from the PRIA Coalition and from a group of non-governmental organizations. Those comments are summarized in Attachment C, along with EPA’s actions taken in response to the comments.
In addition to the public notice that EPA published in the Federal Register concerning this ICR, EPA staff contacted appropriate stakeholders and asked them for their assessment of the regulatory burden estimates expressed by the Agency in this ICR. The parties consulted represented a range of affected stakeholders (small companies, large companies, agricultural companies, antimicrobial companies, and a coalition of trade associations). The specific entities consulted were Ecolab, Syngenta, the PRIA Coalition, Lallemand Plant Care, Pyxis Regulatory Consulting, Clorox, Bengal Labs, Synergy, and Albaugh LLC. The consultation questionnaire is included as Attachment A.
Pyxis Regulatory Consulting’s response to the questionnaire is included as Attachment B. EPA’s response to Pyxis is included in the Response to Comments in Attachment C.
9. Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
No payments or gifts are provided to respondents.
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy. If the collection requires a system of records notice (SORN) or privacy impact assessment (PIA), those should be cited and described here.
None of the information collected by EPA under this ICR comprises confidential business information (CBI).
Bilingual labeling verification submitted by pesticide registrants under this ICR is considered by the EPA Office of Pesticide Programs (OPP) to contain no CBI. EPA does not anticipate that the self-verification of bilingual labeling compliance will contain any CBI. If respondents submit data that contains CBI or relates to trade secrets or commercial or financial information, such information is protected from disclosure under section 10 of FIFRA.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
The information collection activities do not include questions of a sensitive nature.
12. Provide estimates of the hour burden of the collection of information.
Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. Unless directed to do so, agencies should not conduct special surveys to obtain information on which to base hour burden estimates. Consultation with a sample (fewer than 10) of potential respondents is desirable. If the hour burden on respondents is expected to vary widely because of differences in activity, size, or complexity, show the range of estimated hour burden, and explain the reasons for the variance. Generally, estimates should not include burden hours for customary and usual business practices.
If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens.
Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories. The cost of contracting out or paying outside parties for information collection activities should not be included here. Instead, this cost should be included under ‘Annual Cost to Federal Government.’
The North American Industrial Classification System (NAICS) codes assigned to the parties responding to this information collection are as follows:
NAICS Code |
Category |
Description |
325320 |
Pesticide and other agricultural chemical manufacturing |
Individuals or entities engaged in activities related to the registration of a pesticide product. |
325180 |
Other Basic Inorganic Chemical Manufacturing |
Manufacturers of inorganic chemicals used as inert ingredients in pesticide products. |
325199 |
Other Basic Organic Chemical Manufacturing |
Manufacturers of organic chemicals used as inert ingredients in pesticide products. |
96140 |
Regulation of Agricultural Marketing and Commodities |
Includes government establishments responsible for agricultural pest and weed regulation. |
Number of Respondents and Responses
By 2030, every pesticide registrant with an active pesticide registration will be asked to provide information on the incorporation of the required bilingual labeling for each of their pesticide products. There are about 1,820 pesticide registrants with active registrations, and there are a total of about 19,620 registered pesticide products. For the period covered by this ICR, the number of respondents is shown in Table 1 below and ranges from about 600 in the first year to about 1,500 in the third year.
Table 1. Number of Products and Registrants for Which Bilingual Labeling is Required by Year |
|||||||
Calendar Year |
Number of Products |
Total Products Requiring Self Verification |
Number of Registrants |
Average Number of Products per Registrant |
|||
RUPs1 |
Agricultural Tox I |
Non-Ag Tox I |
Ag Tox II |
||||
2025 |
977 |
1,176 |
|
|
2,153 |
601 |
3.6 |
2026 |
977 |
1,176 |
3,157 |
|
5,310 |
1,339 |
4.0 |
2027 |
977 |
1,176 |
3,157 |
1,119 |
6,429 |
1,517 |
4.2 |
Source: EPA estimates based on number of registered products in each category. |
|||||||
Frequency of Response
Registrant response per product is once per year. (Note that, while respondents will not need to update their responses in MyPeST every year for every product – registrants do not need to re-check checkboxes or recertify responses every year, provided they have made no labeling changes that result in a new compliance date nor released products for shipment that previously were not released – EPA is making a conservative burden estimate and assuming that annual updates might be necessary.)
Annual Burden, per Pesticide Product
For each product, the respondent needs to:
Read the MyPeST instructions (read instructions)
Determine what parts of the labeling need translation and enroll in MyPeST if necessary (plan activities)
Retrieve a copy of the labeling (gather information)
Evaluate if the labeling is compliant (gather information)
Log in to MyPeST and check box on the MyPeST application (record information)
Read instructions: To begin the self-verification, respondents will need to read the instructions. There will be new MyPeST instructions that the Agency disseminates specifically about bilingual labeling. These instructions will require respondents to check boxes on the MyPeST application, indicating that the respondents have included bilingual pesticide labeling on the selected products or that the bilingual labeling requirements are not applicable to that product because they will not be released for shipment. The instructions will also require that registrants click a button certifying their responses. EPA estimates that it will take the respondent one hour to read the instructions; time is split between administrative and professional employees.
Plan activities: To determine what parts of the product labeling need translation, respondents will need to consult the Spanish Translation Guide, as all of the parts of the labeling that are within the guide will need translation. Respondents will also need to determine whether their company is enrolled in MyPeST, and if not, complete a one-time registration. EPA estimates this will take the respondent approximately 30 minutes of professional time. Note that, while the burden is calculated per product, a registrant only needs to enroll in MyPeST once for all of their products; the burden thus represents a conservative estimate.
Gather information: The respondent will need to retrieve a copy of the current labeling and evaluate the labeling to see if it is compliant with the PRIA 5 requirements. Retrieving an electronic copy of the labeling is estimated to take 30 minutes of administrative time but determining whether the labeling is compliant is estimated to take an average of two hours of professional time per end use product. The time required may vary somewhat depending on the complexity of the labeling, but the parts of the labeling that are translated are consistent across end use products. To be compliant with the PRIA 5 requirements, the parts of the end-use labeling, where translation is available in the EPA Spanish Translation Guide, must include Spanish language translations.
Record and maintain information: The respondent needs to log into MyPeST and record on the MyPeST application whether each product includes bilingual labeling (record information). Respondents may need to consult their responses in MyPeST occasionally throughout the year, to answer questions (maintain information). Registrants may also choose to voluntarily provide the URL for the bilingual labeling, though they are not required to do so to affirm compliance with PRIA 5. Combined, these activities take an estimated 30 minutes of administrative time and 30 minutes of professional time annually.
MyPeST will store responses from year to year, so EPA does not anticipate that respondents will keep separate records.
The cost of translating and printing labeling is also not included, as those are requirements of PRIA 5, not this data collection.
For each product, the burden hours and cost estimates are provided in Table 2. EPA estimated the burden hours based on a similar ICR, specifically the burden associated with completing a form for pesticide registration maintenance fees, which also requires gathering and recording information about individual products. Hourly wages are from the Bureau of Labor Statistics fully loaded wage rates for administrative and professional employees for 2024.
Table 2: Respondent Estimated Burden per Registered Product2 |
||||
Activity |
Administrative Hours |
Professional Hours |
||
Burden Hours (per Year) |
Cost ($61.31 per hour) |
Burden Hours (per Year) |
Cost ($166.18 per hour) |
|
1. Read instructions |
0.5 |
$31 |
0.5 |
$83 |
2. Plan activities |
0 |
$0 |
0.5 |
$83 |
3. Gather information |
0.5 |
$31 |
2 |
$332 |
4. Record and maintain information |
0.5 |
$31 |
0.5 |
$83 |
Total Burden* |
1.5 |
$92 |
3.5 |
$582 |
*Note: Numbers may not sum due to rounding.
EPA conservatively estimates that there will be an average burden of five hours and $674 per product. For the purposes of this ICR, we are assuming the burden per product stays the same over time. In practice, it is likely that the burden to verify every year will be lower after the first certification.
Total Annual Burden
The total burden associated with this ICR will be the burden per product multiplied by the number of products required to contain bilingual labeling. As described above, for the duration of this ICR, only some of these products will require registrants to self-verify that they have complied with the PRIA 5 bilingual labeling requirements. In year one of this ICR, only restricted use pesticides and agricultural pesticides classified as Toxicity Category I require self-verification. In year two those same products in year one plus non-agricultural pesticides and antimicrobial products classified as Toxicity Category I require self-verification, and in year three all the products from year two plus agricultural pesticides classified as Toxicity Category II. The deadline for other products to be self-verified is beyond the expiration date of this ICR. In the first year, there are 2,153 registered products required to self-verify, 5,310 products in the second year and 6,429 products in the third year, as shown in Table 1 above.
The total burden of the self-verification is the estimate of the average burden per product of five hours and $674 from Table 2 multiplied by the number of products from Table 1. The burden per year of the self-verification requirement is shown in Table 3.
Table 3. Burden of Self-Verification by Year |
|||||
Calendar Year |
RUPs |
Agricultural Tox I |
Non-Ag Tox I |
Ag Tox II |
Total |
Annual Burden (Hours) |
|||||
2025 |
5,862 |
7,056 |
|
|
12,918 |
2026 |
5,862 |
7,056 |
18,942 |
|
31,860 |
2027 |
5,862 |
7,056 |
18,942 |
6,714 |
38,574 |
Total Annual Average |
27,784 |
||||
Annual Burden (Cost) |
|||||
2025 |
$769,876 |
$926,688 |
|
|
$1,696,564 |
2026 |
$769,876 |
$926,688 |
$2,487,716 |
|
$4,184,280 |
2027 |
$769,876 |
$926,688 |
$2,487,716 |
$881,772 |
$5,066,052 |
Total Annual Average |
$3,648,965 |
||||
Source: EPA estimates based on the burden estimates in Table 1 and the number of product estimates in Table 2. |
|
||||
Average Annual Burden, per Respondent
The average burden per respondent is estimated in Table 4. To estimate the average burden per registrant, we divide the total burden (Table 3) by the number of registrants. The top panel of Table 4 shows the number of registrants in each category that requires self-verification. Because RUPs can be also counted in other categories, e.g., Agricultural Tox I, the number of registrants in other columns includes only those with non-RUP products. The average burden per registrant in hours is shown in the middle panel of Table 4 and the average monetary burden is shown in the bottom panel.
Table 4. Average Burden of Self-Verification by Year |
|||||
Calendar Year |
RUPs |
Agricultural Tox I |
Non-Ag Tox I |
Ag Tox II |
Total |
Number of Registrants Required to Self-Verify |
|||||
2025 |
222 |
379 |
|
|
601 |
2026 |
222 |
379 |
738 |
|
1,339 |
2027 |
222 |
379 |
738 |
178 |
1,517 |
Burden per Registrant, Hours |
|||||
2025 |
26 |
19 |
|
|
21 |
2026 |
26 |
19 |
26 |
|
24 |
2027 |
26 |
19 |
26 |
38 |
25 |
Burden per Registrant, Cost |
|||||
2025 |
$3,470 |
$2,445 |
|
|
$2,824 |
2026 |
$3,470 |
$2,445 |
$3,369 |
|
$3,124 |
2027 |
$3,470 |
$2,445 |
$3,369 |
$4,954 |
$3,339 |
Source: EPA estimates, based on the burden estimates in Table 3 and the product estimates in Table 2. |
|
||||
Total Annualized Burden
Table 5 shows the present value and annualized cost to registrants for self-verification. For registrants, the only change over time is the number of products that need self-verification. The present value is calculated over three years as
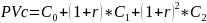
Where PVc is the present value of the cost of self-verification, Ct is the total cost of self-verification in year t (0, 1 or 2) as shown in Table 3, and r is the discount rate. The discount rates reflect anticipated inflation over the three-year period of the ICR. The present value shown in Table 6 is calculated with discount rate r of both 3% and 7%.
The annualized cost is given by the formula
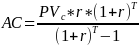
Where AC represents the annualized costs, and T is maximum period of analysis, or T=3 in this case. The annualized cost is shown for both 3% and 7% discount rates. For both the present value and the annualized cost estimates, there are no fixed costs for self-verification.
Table 5. Burden of Self-verification |
||
Discount Rate |
3% |
7% |
Present Value of Costs |
$10,534,000 |
$10,032,000 |
Annualized Value of Costs |
$3,724,000 |
$3,823,000 |
Source: EPA estimates |
||
13. Provide an estimate for the total annual cost burden to respondents or recordkeepers resulting from the collection of information.
The cost estimate should be split into two components: (a) a total capital and start-up cost component (annualized over its expected useful life) and (b) a total operation and maintenance and purchase of services component. The estimates should take into account costs associated with generating, maintaining, and disclosing or providing the information. Include descriptions of methods used to estimate major cost factors including system and technology acquisition, expected useful life of capital equipment, the discount rate(s), and the time period over which costs will be incurred. Capital and start-up costs include, among other items, preparations for collecting information such as purchasing computers and software; monitoring, sampling, drilling and testing equipment; and record storage facilities.
If cost estimates are expected to vary widely, agencies should present ranges of cost burdens and explain the reasons for the variance. The cost of purchasing or contracting out information collections services should be a part of this cost burden estimate. In developing cost burden estimates, agencies may consult with a sample of respondents (fewer than 10), utilize the 60-day pre-OMB submission public comment process and use existing economic or regulatory impact analysis associated with the rulemaking containing the information collection, as appropriate.
Generally, estimates should not include purchases of equipment or services, or portions thereof, made: (1) prior to October 1, 1995, (2) to achieve regulatory compliance with requirements not associated with the information collection, (3) for reasons other than to provide information or keep records for the government, or (4) as part of customary and usual business or private practices.
There are no capital and/or maintenance costs for this collection.
14. Provide estimates of annualized cost to the Federal government. Also, provide a description of the method used to estimate cost, which should include quantification of hours, operational expenses (such as equipment, overhead, printing, and support staff), and any other expense that would not have been incurred without this collection of information. Agencies may also aggregate cost estimates from Items 12, 13, and 14 in a single table.
The Federal costs for this information collection are shown in Table 6. Unlike the costs to respondents, there are significant startup costs to EPA. While the number of products that need to confirm bilingual labeling change each year, after the startup costs are accounted for, the costs per year for notifying respondents is the same, regardless of how many products are required to have bilingual labeling. That is because these processes will be automated, once the systems are in place.
To begin collecting information on the prevalence of the required bilingual labeling, EPA has several startup tasks. First, determining whether the product is an RUP, its toxicity category, and whether it is an agricultural or antimicrobial pesticide, is necessary to determine whether bilingual labeling is required during the three years covered by this ICR. Therefore, some database work will need to be completed in the first year to identify the number of RUP agricultural pesticides, toxicity category 1 agricultural pesticides, and toxicity category 1 antimicrobial products so that this information can be displayed in MyPeST. EPA estimates that 40 hours of professional time will be required in year one for the database work, as well as drafting an explanation of the reporting process. Second, the MyPeST application, which is available to every registrant, must be programmed to include information about the pesticide product type (e.g., RUP) and checkboxes marked “Bilingual Labeling” and “Not Released for Shipment” beside them, for the respondent to indicate whether the product is in compliance with the requirements when they submit the information to EPA. EPA is spending $60,000 for a contract to make these changes. In addition, managers in four divisions of the Office of Pesticide Programs and the Immediate Office are estimated to spend an hour each reviewing the MyPeST changes, and staff are estimated to spend another 2 hours to make final revisions, for a total of 5 hours of managerial time and 2 hours of professional time. These are one-time costs.
In order that respondents know to enter information on the application in the first year, EPA must inform them that the Agency will use MyPeST to collect information on bilingual labeling compliance and send the reporting instructions. EPA has multiple ways of informing respondents, including the OPP Update Listserv, stakeholder meetings, and the MyPeST application itself. EPA estimates spending 20 hours of staff time and 20 hours of managerial time in the Office of Pesticide Programs preparing, revising, and conveying external communications.
Once EPA receives the completed information from the respondents in MyPeST, EPA will tabulate the information and make public the results. This is a new activity. EPA will calculate the number of products with bilingual labeling by category, such as RUPs, Toxicity Category I agricultural pesticides, etc., and present the results on a public-facing EPA website. These activities will take place every year. EPA estimates that compiling and presenting the information will take about 12 hours of professional time, as shown in Table 6 below.
Costs are estimated using hourly wages of federal employees from the Bureau of Labor Statistics, fully loaded wage to account for benefits and overhead.
T Table 6. Agency Burden and Total Cost |
||||||
Agency Activities |
Burden (Hours) |
Total per Activity |
||||
Technical |
Clerical |
Managerial |
Hours |
Cost (Dollars) |
||
$101.75 per hour |
$55.02 per hour |
$151.07 per hour |
||||
Year 1 |
||||||
Review databases for product information, create explanation of reporting process |
40 |
2 |
|
42 |
$4,180 |
|
Contractor creates MyPeST features |
|
|
|
|
$60,000 |
|
Review MyPeST changes |
2 |
|
5 |
7 |
$959 |
|
Inform registrants of new reporting system |
20 |
|
20 |
40 |
$5,056 |
|
Tabulate Responses |
8 |
2 |
2 |
12 |
$1,226 |
|
Total, Year 1 |
70 |
4 |
27 |
101 |
$71,421 |
|
|
||||||
Tabulate Responses, Year 2 |
8 |
2 |
2 |
12 |
$1,226 |
|
Tabulate Responses, Year 3 |
8 |
2 |
2 |
12 |
$1,226 |
|
3-year Total |
125 |
$73,874 |
||||
Annual Average |
42 |
$24,625 |
||||
|
||||||
|
3% Discount Rate |
7% Discount Rate |
||||
Present Value of Agency Costs |
$73,768 |
$73,638 |
||||
Annualized Agency Costs |
$26,079 |
$28,060 |
||||
Source: Agency estimates. Wages derived from Bureau of Labor Statistics (http://www.bls.gov/oes/current/naics4_999100.htm). NAICS 999100 – Federal Executive Branch. Standard Occupational Codes (SOC): 11-0000 for management occupations, 19-0000 for technical occupations, and 43-0000 for clerical occupations.
The present value of the Agency burden and the annualized cost are also shown in Table 6. The present value of Agency costs over the three years covered by this ICR, accounting for the initial costs for contracting modifications to MyPest, ranges from $73,768 at a 3% discount rate to $73,638 at a 7% discount rate. The annualized costs range from $38,552 to $40,729, respectively. Because most of these costs occur at the beginning of the tracking project, these costs will be lower in future years.
15. Explain the reasons for any program changes or adjustments reported on the burden worksheet.
This is a new information collection request, so there are no changes or adjustments.
16. For collections whose results will be published, outline the plans for tabulation and publication. Address any complex analytical techniques that will be used. Provide the time schedule for the entire project, including beginning and ending dates of the collection of information, completion of report, publication dates, and other actions.
Information from this collection will be presented on an EPA web page. EPA is required to make the tracking of bilingual labeling publicly available. The published results will not need any complex analytical techniques, as they are simply counts of self-verification and percentages of products that are in compliance with PRIA 5.
The time schedule will begin when EPA transmits the instructions to respondents. The information is required to be entered into MyPeST by January 28th every year, except for the first year; reporting on compliance with the December 2025 deadline is required by June 28, 2026. After EPA receives the information in MyPeST, calculations and transmittal of results will be completed within six months.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons why display would be inappropriate.
The entry of information into MyPeST needs no expiration date, as this information request will be ongoing as required by PRIA 5, so there is no expiration of the requirement.
18. Explain each exception to the topics of the certification statement identified in “Certification for Paperwork Reduction Act Submissions.”
This information collection complies with all provisions of the Certification for Paperwork Reduction Act Submissions.
SUPPLEMENTAL INFORMATION
PRA Burden Statement
This collection of information is approved by OMB under the Paperwork Reduction Act, 44 U.S.C. 3501 et seq. (OMB Control No. 2070-NEW). Responses to this collection of information are mandatory for certain persons, as specified at 40 CFR Part 152. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The public reporting and recordkeeping burden for this collection of information is estimated to be 5 hours per response. Send comments on the Agency’s need for this information, the accuracy of the provided burden estimates and any suggested methods for minimizing respondent burden to the Data & Enterprises Programs Division Deputy Director, U.S. Environmental Protection Agency (2821T), 1200 Pennsylvania Ave., NW, Washington, D.C. 20460. Include the OMB control number in any correspondence. Do not send the completed form to this address.
You can also provide comments to the Office of Information and Regulatory Affairs, Office of Management and Budget via https://www.reginfo.gov/public/do/PRAMain. Find this particular information collection by selecting ‘‘Currently under 30-day Review—Open for Public Comments’’ or by using the search function.
All comments received by EPA will be included in the docket without change, including any personal information provided, unless the comment includes profanity, threats, information claimed to be Confidential Business Information (CBI), or other information whose disclosure is restricted by statute. Do not submit electronically any information you consider to be CBI or other information whose disclosure is restricted by statute.
LIST OF ATTACHMENTS
The attachments listed below can be found in the docket for this ICR or by using the hyperlink that is provided in the list below. The docket for this ICR is accessible electronically through https://www.regulations.gov using Docket ID Number: EPA-HQ-OPP-2025-0049.
Attachment |
Description |
A |
Stakeholder Consultation Email and Consultation Questions |
B |
Response Received to Stakeholder Consultation Questions |
C |
Responses to Comments Received on Proposed New Information Collection Request (ICR) for Tracking the Adoption of Bilingual Labeling of Pesticide Products |
D |
Mock-Up of MyPeST Interface (PFN 8500-31) |
E |
Selections From MyPeST User Guide: Instructions for Reporting Bilingual Labeling Compliance with PRIA 5 |
F |
Pesticide Registration Notice 2025-2 |
References
40 CFR 152 – Pesticide Registration and Classification Procedures available at https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-152
Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Section 4(i)(5), pages 57-61 available at https://www.govinfo.gov/content/pkg/COMPS-10326/pdf/COMPS-10326.pdf
Pesticide Registration Improvement Act (PRIA) 5 available at https://www.govinfo.gov/content/pkg/PLAW-117publ328/html/PLAW-117publ328.htm
1 Restricted Use Products (RUPs) when used requires the product to be applied by a certified applicator or someone under the direct supervision of a certified applicator.
2
The wage costs per hour in Table 2 are based on 2024 data from the
Bureau of Labor Statistics fully loaded wage rates for
administrative and professional employees of chemical manufacturing
and registrants. These wage rates vary by industry. For Table 1, we
use the wage rates based on unloaded
wage data sources:
Bureau
of Labor Statistics
NAICS
3250A1 - Chemical Manufacturing (3251, 3252, 3253, and 3259 only).
Standard Occupational Codes (SOC): 11-0000 for management
occupations, 19-0000 for technical occupations, and 43-0000 for
office and administrative support occupations.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Gross, Isabel |
| File Modified | 0000-00-00 |
| File Created | 2026-01-01 |
© 2026 OMB.report | Privacy Policy